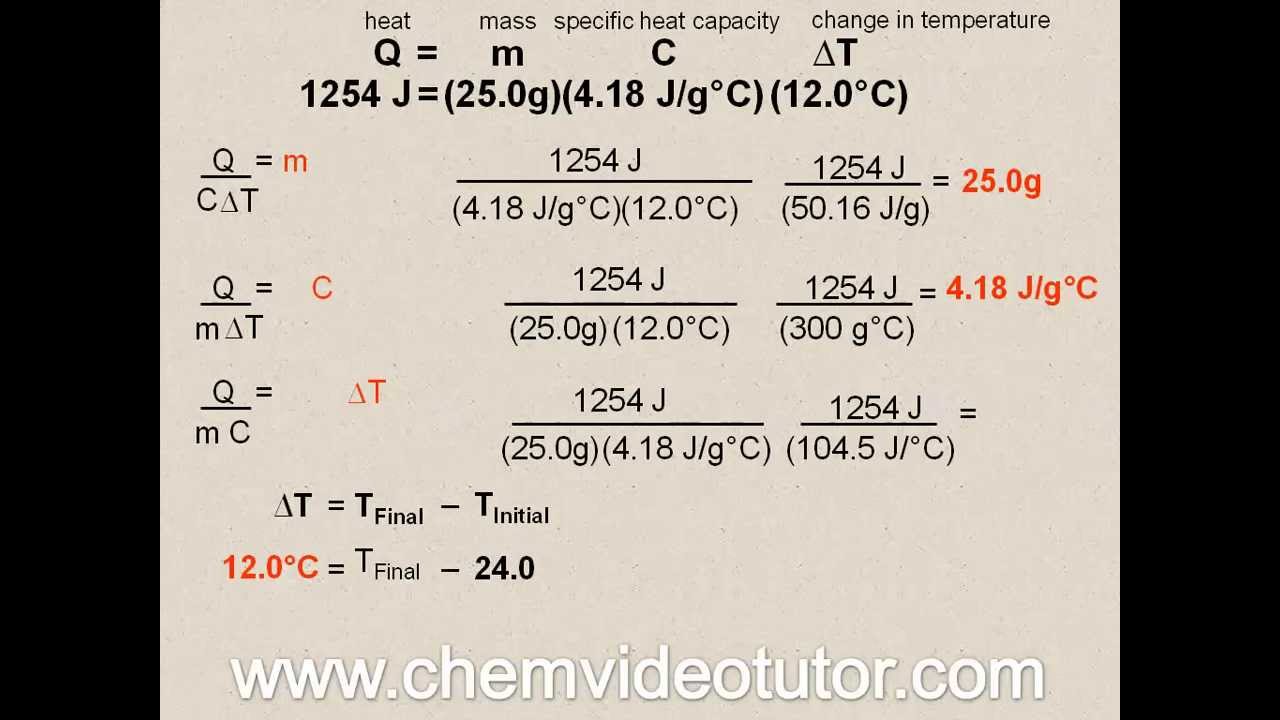
What Does C Stand For In Q=Mct . Heat energy = mass × specific heat × change in temperature q =. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. heat (q) is the transfer of thermal energy between two systems at different temperatures. Q = mcδt, where q is the. the heat capacity of an object made of a pure substance is, c=mc. The quantitative relationship between heat transfer and temperature change contains. the quantitative relationship between heat transfer and temperature change contains all three factors: The formula q = mct is used to calculate heat energy. Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. heat transfer and energy change. If the material an object is made of is uniform in. Heat is calculated using the formula q=mcδt.
from www.youtube.com
Heat energy = mass × specific heat × change in temperature q =. Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. Q = mcδt, where q is the. heat transfer and energy change. the quantitative relationship between heat transfer and temperature change contains all three factors: If the material an object is made of is uniform in. Heat is calculated using the formula q=mcδt. the heat capacity of an object made of a pure substance is, c=mc. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. The quantitative relationship between heat transfer and temperature change contains.
Chemistry Regents Advanced Heat Calculations using Q=mCΔT YouTube
What Does C Stand For In Q=Mct Heat energy = mass × specific heat × change in temperature q =. heat (q) is the transfer of thermal energy between two systems at different temperatures. The quantitative relationship between heat transfer and temperature change contains. Heat is calculated using the formula q=mcδt. If the material an object is made of is uniform in. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. The formula q = mct is used to calculate heat energy. the quantitative relationship between heat transfer and temperature change contains all three factors: heat transfer and energy change. Q = mcδt, where q is the. Heat energy = mass × specific heat × change in temperature q =. Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. the heat capacity of an object made of a pure substance is, c=mc.
From www.chegg.com
Solved Q mcAT Equation 2 1. Show that the units of specific What Does C Stand For In Q=Mct The formula q = mct is used to calculate heat energy. Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. Heat energy = mass × specific heat × change in temperature q =. the quantitative relationship between heat transfer and temperature change contains. What Does C Stand For In Q=Mct.
From www.youtube.com
Specific Heat Capacity of Water q = mct Chemistry (Part 2 of 3) YouTube What Does C Stand For In Q=Mct The formula q = mct is used to calculate heat energy. Heat is calculated using the formula q=mcδt. heat transfer and energy change. Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. the heat capacity of an object made of a pure. What Does C Stand For In Q=Mct.
From www.youtube.com
11.2 q=mCT YouTube What Does C Stand For In Q=Mct Q = mcδt, where q is the. heat transfer and energy change. heat (q) is the transfer of thermal energy between two systems at different temperatures. the quantitative relationship between heat transfer and temperature change contains all three factors: Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units. What Does C Stand For In Q=Mct.
From www.youtube.com
R1.1.4 Calculating ΔH using q = mcΔT YouTube What Does C Stand For In Q=Mct Q = mcδt, where q is the. heat (q) is the transfer of thermal energy between two systems at different temperatures. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. the quantitative relationship between heat transfer and temperature change contains all three factors: Heat energy =. What Does C Stand For In Q=Mct.
From www.youtube.com
(q=mcT) how to find the Enthalpy change in a chemical reaction What Does C Stand For In Q=Mct Heat energy = mass × specific heat × change in temperature q =. If the material an object is made of is uniform in. Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. the heat capacity of an object made of a pure. What Does C Stand For In Q=Mct.
From www.slideshare.net
Q=mc t What Does C Stand For In Q=Mct the quantitative relationship between heat transfer and temperature change contains all three factors: The quantitative relationship between heat transfer and temperature change contains. the heat capacity of an object made of a pure substance is, c=mc. The formula q = mct is used to calculate heat energy. Heat is calculated using the formula q=mcδt. Heat energy = mass. What Does C Stand For In Q=Mct.
From www.youtube.com
How to Solve for Heat (Q) Released in a Reaction using Q=mcT Equation What Does C Stand For In Q=Mct If the material an object is made of is uniform in. Heat energy = mass × specific heat × change in temperature q =. Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. heat transfer and energy change. the heat capacity of. What Does C Stand For In Q=Mct.
From www.youtube.com
Unit 6 Lesson 5 q=mcdeltaT YouTube What Does C Stand For In Q=Mct the heat capacity of an object made of a pure substance is, c=mc. Heat energy = mass × specific heat × change in temperature q =. Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. the heat capacity formula is expressed as. What Does C Stand For In Q=Mct.
From f0rmule.blogspot.com
Formule Q = M.c.delta T Formule What Does C Stand For In Q=Mct Heat energy = mass × specific heat × change in temperature q =. Heat is calculated using the formula q=mcδt. the heat capacity of an object made of a pure substance is, c=mc. Q = mcδt, where q is the. heat transfer and energy change. Q = heat energy (joules, j) m = mass of a substance (kg). What Does C Stand For In Q=Mct.
From www.youtube.com
Chemistry Regents Advanced Heat Calculations using Q=mCΔT YouTube What Does C Stand For In Q=Mct Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. Q = mcδt, where q is the. The formula q = mct is used to calculate heat energy. the quantitative relationship between heat transfer and temperature change contains all three factors: Heat is calculated. What Does C Stand For In Q=Mct.
From www.showme.com
Solving q=mcat Science, Chemistry ShowMe What Does C Stand For In Q=Mct The quantitative relationship between heat transfer and temperature change contains. heat (q) is the transfer of thermal energy between two systems at different temperatures. The formula q = mct is used to calculate heat energy. Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning. What Does C Stand For In Q=Mct.
From www.youtube.com
2.0 Level q=mc∆T Practice YouTube What Does C Stand For In Q=Mct If the material an object is made of is uniform in. Q = mcδt, where q is the. The formula q = mct is used to calculate heat energy. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. Heat is calculated using the formula q=mcδt. Heat energy =. What Does C Stand For In Q=Mct.
From www.madebyteachers.com
Heat Transfer Calorimetry q = mCT First Law of Thermodynamics Worksheet What Does C Stand For In Q=Mct the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. Heat is calculated using the formula q=mcδt. the quantitative relationship between heat transfer and temperature change contains all three factors: the heat capacity of an object made of a pure substance is, c=mc. The quantitative relationship between. What Does C Stand For In Q=Mct.
From www.youtube.com
Specific Heat Capacity (q=mC∆T) Examples, Practice Problems, Initial What Does C Stand For In Q=Mct Heat is calculated using the formula q=mcδt. The formula q = mct is used to calculate heat energy. heat transfer and energy change. Q = mcδt, where q is the. the heat capacity of an object made of a pure substance is, c=mc. The quantitative relationship between heat transfer and temperature change contains. Q = heat energy (joules,. What Does C Stand For In Q=Mct.
From www.youtube.com
"Q=mcat" YouTube What Does C Stand For In Q=Mct If the material an object is made of is uniform in. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. heat transfer and energy change. Heat energy = mass × specific heat × change in temperature q =. heat (q) is the transfer of thermal energy. What Does C Stand For In Q=Mct.
From worksheetfullfunniest.z21.web.core.windows.net
How To Calculate Heat Chemistry What Does C Stand For In Q=Mct The formula q = mct is used to calculate heat energy. Heat is calculated using the formula q=mcδt. the quantitative relationship between heat transfer and temperature change contains all three factors: the heat capacity of an object made of a pure substance is, c=mc. Q = heat energy (joules, j) m = mass of a substance (kg) c. What Does C Stand For In Q=Mct.
From www.youtube.com
q=mcT homework 1 YouTube What Does C Stand For In Q=Mct Q = mcδt, where q is the. Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. The quantitative relationship between heat transfer and. What Does C Stand For In Q=Mct.
From www.youtube.com
Calorimetry Specific Heat Capacity q = mct MADE SUPER SIMPLE Thermal What Does C Stand For In Q=Mct Heat energy = mass × specific heat × change in temperature q =. the heat capacity formula is expressed as the product of mass, specific heat, and change in the temperature which is. Q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. . What Does C Stand For In Q=Mct.